
More about Thermal Cracking
 |
 |
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
The main reaction in thermal cracking is the reverse of free-radical polymerisation - instead of small compounds with carbon-carbon double bonds combining to form a long chain, relatively long chains are decomposed to give compounds with carbon-carbon double bonds.
This is why it is impossible to form polymers by free-radical addition at very high temperatures - above a certain temperature, called the ceiling temperature, this depropagation reaction is favoured over chain growth.
The key reactions in thermal cracking are shown below:
 An initiation reaction - homolysis of a carbon-carbon bond (only spontaneous at high temperatures). (Homolysis is where the bond breaks so that each fragment has one electron) |
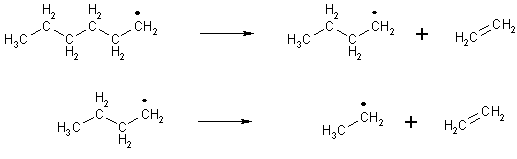 Some depropagation reactions in thermal cracking |
 A chain transfer reaction - rapid transfer of hydrogens creates a large variety of reactive species |
 A termination reaction - transfer of hydrogen between two free-radical species can end the chain |
 |
 |
 |