
Bakelite Synthesis
 |
 |
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
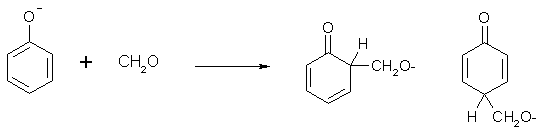
It is not actually the phenol but the phenoxide ion (formed due to the weak acidity of phenol) that is the reactive species.
The electron-deprived C of formaldehyde adds to the phenol ortho or para to the oxygen, since these are the spots where resonance structures can be drawn to give a formal negative charge.
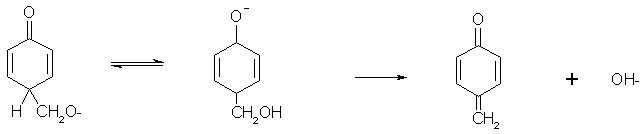
We can lose OH- from the substituent (so we end up with one H2O per formaldehyde, and a technical condensation).
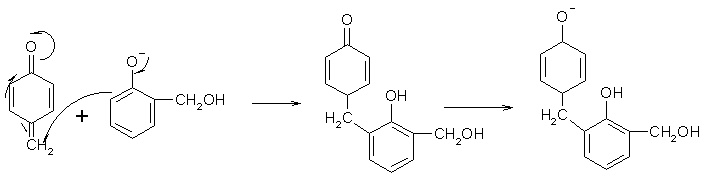
Then there are a lot of arrows moving around, and what it looks like is a carbanion addition to a double bond....
 |
 |
 |