|
Students learn to account for the cleaning action of soap by describing its molecular structure.
The cleaning action of soaps and detergents is based on the property
known as detergency. To remove oils and greases, soaps and detergents
help make an emulsion. To illustrate this, let's look at how you clean
a greasy plate.
|
You take the plate with some grease on it and dip it in sudsy water
(a detergent solution), then agitate.
|

|
|
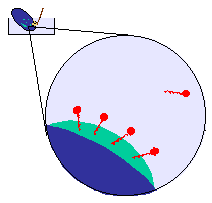
At the molecular level the detergent molecules are adsorbing onto
the grease,
|
|
|
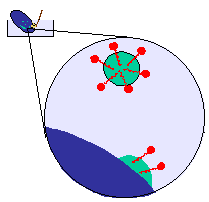
and your agitation or scrubbing is breaking the grease into small droplets.
|
|
|
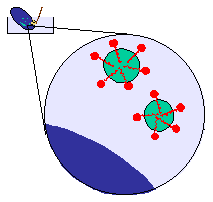
The role of the detergent is to prevent the droplets coalescing and
redepositing on the plate.
|
|
The cleaning action of soaps and detergents is based on the
surface-active nature of the molecules. The molecular structure
of the surfactant is what allows it to help us clean.
|




















