
Chemistry of Fermentation
 |
 |
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |
|
Students learn to summarise the chemistry of the fermentation process
Chemical Reactions in Fermentation
The fermentation of sugars to ethanol was not a reaction invented by micro-organisms for our benefit; it has a venerable pedigree extending back billions of years, to the time before our planet had such an oxygen-rich atmosphere.
Essentially, fermentation is a means for organisms to extract energy from their chemical environment. The diagram below shows a small section of an incredibly complex net of biochemical processes that go on in the cells that do the work of ethanol synthesis for us.
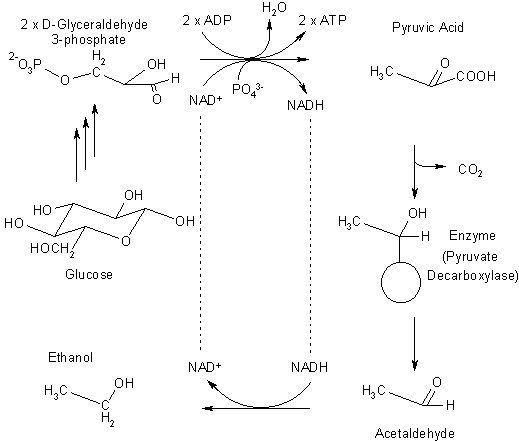 One possible biochemical pathway (the one used by both yeast and humans) for the fermentation of glucose to ethanol and carbon dioxide |
The reaction starts on the left hand side, with glucose, the main energy source for humans and brewers' yeast. A series of enzyme-catalysed reactions that are common to both of these species converts each molecule of glucose into two molecules of glyceraldehyde-3-phosphate.
In humans and in yeast, this molecule is converted to the carboxylic acid pyruvic acid, generating two molecules of adenosine triphosphate (ATP), the body's energy currency, in the process. We either convert the pyruvic acid to lactic acid in our muscle tissue (a reaction that only extracts a small fraction of the possible energy, but does it quickly for when we need energy fast) or break it all the way down to carbon dioxide and water with oxygen in a long series of reactions that provide most of our energy needs.
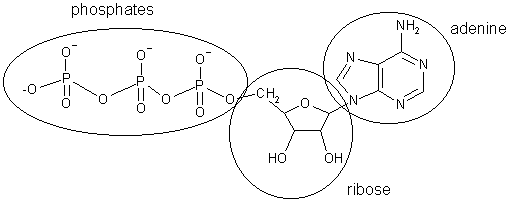
Adenosine triphosphate, the body's energy currency. It is made up of adenine (one of the bases found in DNA) linked to ribose (almost deoxyribose, the sugar found in DNA) to form adenosine, with three phosphate units attached to a hydroxy group of the ribose. |
Yeast lack the enzymes that allow us to use the lactic acid pathway, but their lives are slow and simple, and they can get by with the little bit of energy provided by pyruvic acid formation if there is no oxygen around. We, unfortunately, don't have that choice.
The fermentation steps are the conversion of pyruvic acid to acetaldehyde, and then to ethanol. The hydrogen required for the conversion of acetaldehyde to ethanol is provided by nicotinamide adenine dinucleotide (NADH); the resulting hydrogen-deprived NAD+ is necessary to drive the covnersion of D-glyceraldehyde-3-phosphate to pyruvic acid, ensuring the cycle keeps turning around. (We mammals have different ways to get NAD+, which don't work well in the absence of oxygen).
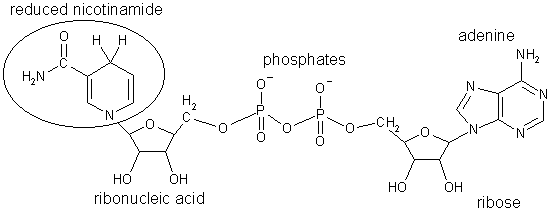
Nicotinamide adenine dinucleotide, an essential cofactor in many enzyme-catalysed reactions (reduced form) |
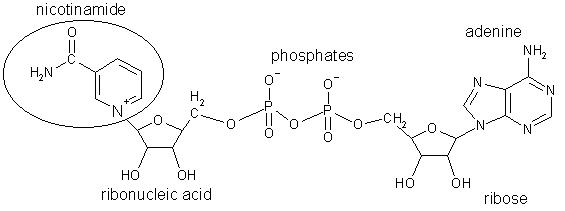
Nicotinamide adenine dinucleotide, an essential cofactor in many enzyme-catalysed reactions. Note that the nitrogen in the nicotinamide is now postively charged, although the molecule has lost a hydrogen. |
The initial steps before fermentation itself, in which starch is converted to glucose, are parallel to the degradation of cellulose to sucrose discussed in section 9.2.2. One enzyme (alpha-amylase) chops the long chains of starch into dimers of glucose, called maltose. Another enzyme (glucoamylase) cuts these maltose units in half to provide glucose.
 A rough diagram of the structure of starch, showing each glucose unit as a ring |
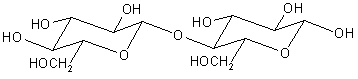 Maltose, two glucose units joined by an alpha-1,4 link. Compare with cellobiose, two glucose units joined by a beta-1,4 link, which is formed by the enzymatic decomposition of cellulose |
Production of glucose from sucrose is even simpler; sucrose is a simple dimer of glucose and fructose, both of which can be used as substrates for fermentation to produce ethanol. The yeast itself produces the enzyme invertase, which splits the sugar in half.
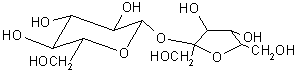 Sucrose, foundation of dentistry. The half on the left is our old friend glucose; on the right, fructose. |
You may have noticed in the description above that the process of fermentation itself gives rise to carbon dioxide; this is captured by the ethanol manufacturers and used to prepare dry-ice for use in other industrial applications. The carbon dioxide produced in this step of the process is actually quite significant if you are considering the total emissions (in terms of greenhouse gases) from the use ethanol as a fuel (compared to some other fuel). We'll look at that in more detail later.
 |
 |
 |